Over the past five decades, antibody therapeutics have undergone a remarkable transformation, from the murine monoclonals of the 1970s to the fully human antibodies that are increasingly paving the way for many successful biologics. This evolution has been driven by a series of pivotal innovations and discoveries: hybridoma technology, camelid-derived VHH domains, chimeric and humanized antibodies, phage display, and fully human antibodies transgenic platforms. Each step has brought the field closer to overcoming immunogenicity and manufacturability barriers, while expanding the therapeutic potential of antibodies.
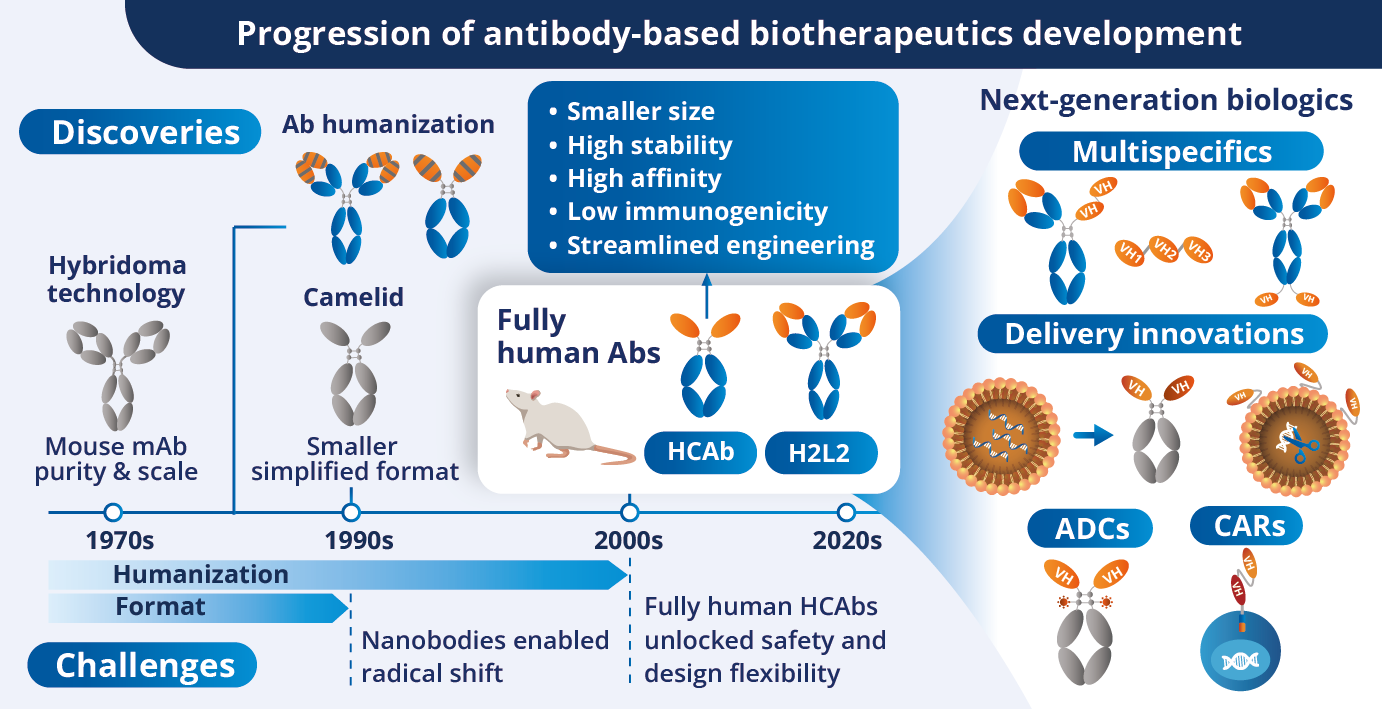
As the clinical landscape progressively shifts toward more modular, multispecific, and cell-engaging formats, the structural limitations of conventional IgG molecules have become increasingly apparent. Notably, their large size and complexity often hinder tissue penetration, limit design flexibility, and complicate manufacturing.
The rise of small, single-domain formats such as camelid-derived VHHs, and more recently, autonomous fully human variable heavy chain (VH) domains has enabled a new wave of innovation centered on compact, highly developable binders.
While murine scFvs and VHHs have enabled the development of novel constructs such as chimeric antigen receptors (CARs) and bispecifics, challenges remain in stability, immunogenicity, and clinical compatibility. Autonomous fully human VH domains, particularly those derived from heavy-chain-only antibodies (HCAbs) via transgenic platforms, offer a compelling alternative. These domains combine the solubility and compactness of VHHs with the human origin essential for clinical translation, delivering high affinity, low aggregation risk, and reduced immunogenicity.
As developers seek to streamline construct design and accelerate IND-enabling studies, fully human VH binders are emerging as a foundational technology for next-generation therapeutics across modalities, from multispecific antibodies to targeted delivery systems.
From Camelid VHH to Human VH Domains
Autonomous variable single domains do not exist naturally but can be isolated from camelid HCAbs [1,2]. Similar to conventional antibodies, single VHH domains from camelid HCAbs exhibit high-affinity antigen-binding specificity.
Due to their compact format and approximately 10-fold smaller molecular size (~15 KDa) compared to conventional IgGs, camelid VHH domains, also known as nanobodies, have rapidly emerged as a versatile platform for biotherapeutic development. Their versatility is readily apparent in the diverse architectures and mechanisms of action of VHH-based biotherapeutics approved to date.
Approved VHH-based biotherapeutics
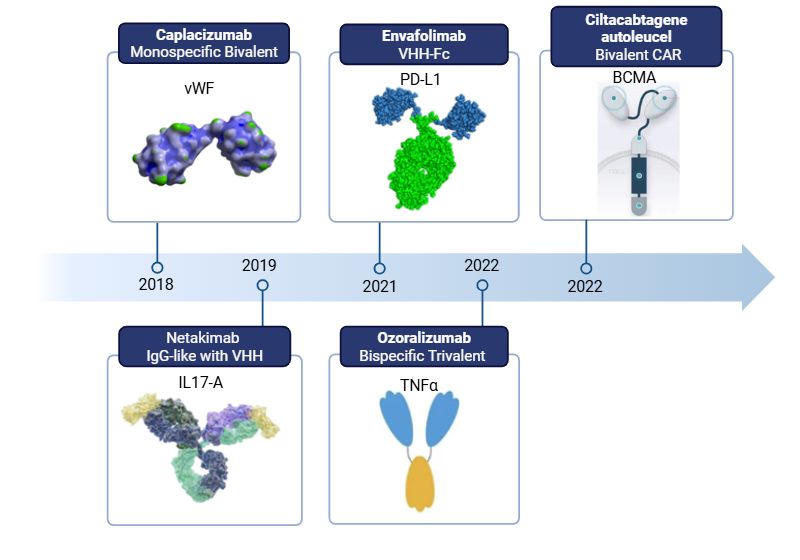
Beyond their small molecular size, VHH domains possess several favorable attributes for biotherapeutic development, including high stability and solubility, low immunogenicity, and a unique capacity to bind hidden epitopes.
Despite the low immunogenicity of camelid VHHs, which is primarily attributed to their high sequence similarity with human immunoglobulin variable genes (i.e., VH3 family), humanization of VHHs is a common practice [3,4]. All approved VHH-based biotherapeutics, as well as most in the clinical pipeline, are humanized to prevent the development of anti-drug antibody (ADA) responses [5].
Different strategies can be employed to humanize VHH domains (e.g., Complementarity Determining Region (CDR) grafting or resurfacing). For example, developers may graft VHH CDR loops onto humanized or fully human framework scaffolds or simply modify key structural residues within framework regions with human germline sequences [6].
However, the outcomes of VHH humanization are varied and sequence specific; improvements in developability properties, such as expression levels and solubility, often come at the cost of reduced binding affinity [5].
Alternatively, to harness the full potential of single-domain binders for next-generation biotherapeutics, developers can leverage fully human VH domains from libraries or established transgenic animals. Among these options, library-sourced VH domains require extensive engineering to achieve optimal developability, which can adversely affect binding affinity and lead to high lead attrition.
In contrast, fully human HCAb VH domains sourced from transgenic animals, such as HCAb Harbour Mice®, offer several advantages, including access to specific high-affinity binders with broad paratope diversity and favorable developability properties (e.g., high stability and low immunogenicity).
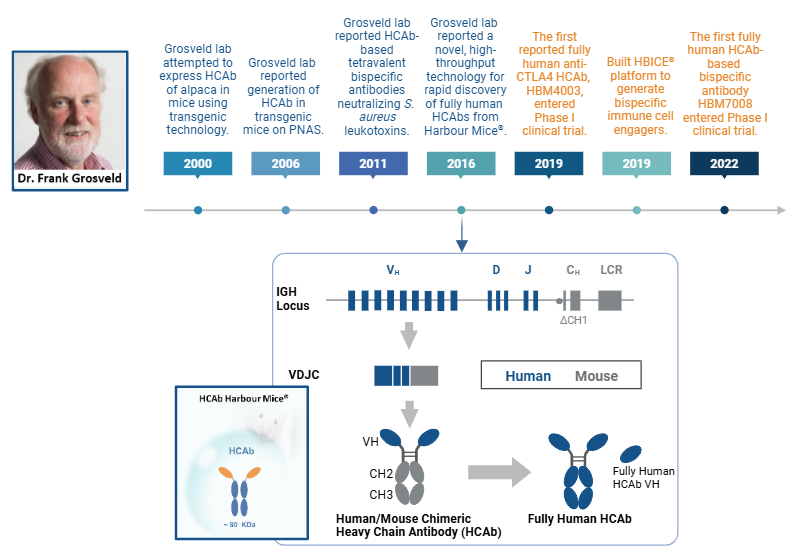
First Fully Human HCAb Transgenic Platform
The foundational work by Drabek et al. 2016 demonstrated the feasibility of generating fully human VH domains using transgenic mice engineered with a human heavy chain-only antibody (HCAb) locus [7,8]. Building on their earlier hybrid llama-human constructs, the team, led by Dr. Frank Grosveld, demonstrated that they could replace camelid VHH segments with four human VH genes, enabling the mice to produce antigen-specific fully human HCAbs upon immunization [8]. These antibodies, isolated and screened using ELISA and biolayer interferometry, showed high affinity, solubility, and no aggregation, with several having desirable functions (i.e., neutralization of influenza virus in vitro). Notably, this study provided the first evidence that functional, monomeric human VH domains with favorable biophysical properties could be reliably sourced from transgenic animals [9].
Expanding on this approach, Dr. Grosveld later developed the HCAb Harbour Mice®, a transgenic mouse line that carries a diverse set of human VH and all D and J gene segments configured to produce HCAbs lacking the CH1 domain. This platform yields high-affinity, soluble, and modular VH domains [9].
Timeline of discoveries and development of HCAb Harbour Mice®
Emergence of Fully Human Single VH Domain-based Biotherapeutics
The favorable attributes of human VH domains sourced from transgenic mice have prompted their use in developing diverse therapeutic modalities. Their compact structure makes them extremely versatile in the design of multispecific therapeutics, supporting unique mechanisms of action and therapeutic goals.
To date, various candidates have advanced to the clinical stage as CAR binders, immune cell engagers (ICEs), and checkpoint inhibitors (CPIs) [9]. Among these, several VH-based therapeutics, sourced from the HCAb Harbour Mice® platform, are currently advancing through clinical studies, including the CPI HBM4003 (anti-CTLA-4) and three bispecific ICEs: HBM7008 (anti-B7H4/anti-4-1BB), HBM7020 (anti-BCMA/anti-CD3), and HBM7022 (AZD5863, anti-CLDN18.2/anti-CD3). This progress underscores the clinical potential of fully human VH domains derived from transgenic platforms.
Fully human single VH domain-based biotherapeutics in the clinical stage
| Therapeutic Name | VH Origin | VH Target | Modality | Indication | Clinical Stage |
|---|---|---|---|---|---|
| CB307 | Transgenic mouse | PSMA, CD137, HSA | ICE PSMAxCD137xHSA | Prostate Cancer | Phase I |
| FHVH33 | Phage display library | BCMA | CAR BCMA | Multiple Myeloma | Phase I |
| GSK1995057 | Phage display library | TNFR1 | Respiratory Disorders | Phase I | |
| GSK2862277 | Phage display library | TNFR1 | Respiratory Disorders | Phase I | |
| HBM4003 | Transgenic mouse | CTLA4 | CPI CTLA4 | Solid Tumors | Phase I/Phase II |
| HBM7008 | Transgenic mouse | 4-1BB | ICE B7H4x4-1BB | Solid Tumors | Phase I |
| HBM7020 | Transgenic mouse | BCMA | ICE BCMAxCD3 | Autoimmune Diseases | Phase I |
| HBM7022 (AZD5863) | Transgenic mouse | CLDN18.2 | ICE CLDN18.2xCD3 | Solid Tumors | Phase I/II |
| TNB-383B | Transgenic mouse | BCMA | ICE BCMAxCD3 | Multiple Myeloma | Phase I/II |
| TNB-486 (AZD0486) | Transgenic mouse | CD19 | ICE CD19xCD3 | B-Cell Non-Hodgkin Lymphoma | Phase I |
| TNB-585 (AMG340) | Transgenic mouse | PSMA | ICE PSMAxCD3 | Prostate Cancer | Phase I |
| TNB-738 | Transgenic mouse | CD38 | CD38 enzyme inhibitor | Endocrinology and Metabolic Disease | Phase I |
Biotherapeutics developed from fully human single VH domains sourced from phage-display libraries and transgenic platforms. Table Ref. 5,9
Progress of the first CPI and ICE from HCAb Harbour Mice® to enter clinical trials
HBM4003
This fully human HCAb monoclonal anti-CTLA-4 was developed to address some efficacy and safety limitations of ipilimumab as the standard of care for several types of cancers. Preclinical findings demonstrated that an HBM4003 candidate, with a modified IgG1 Fc constant domain, exhibited enhanced antibody-dependent cellular cytotoxicity (eADCC) against regulatory T cells (Treg) [10]. In addition to enhanced Treg depletion, HBM4003 demonstrated improved T cell activation, resulting in more efficient tumor growth inhibition. Moreover, Fc domain engineering improved the pharmacokinetics of HBM4003, shortening its half-life, thereby enabling a favorable safety profile as indicated by good tolerability in cynomolgus monkeys [10].
Clinical trials evaluating the efficacy, pharmacokinetics, and tolerability of HBM4003 in combination with the anti-PD-1 antibody toripalimab have been conducted in patients with advanced melanoma [11].
In this phase I trial, HBM4003 plus toripalimab showed manageable safety, low immunogenicity, and promising efficacy in advanced melanoma. While grade ≥3 treatment-related adverse events (TRAEs) occurred in 25% of patients, no grade 4/5 immune-related events (irAEs) were reported. Anti-drug antibodies (ADAs) were detected in only 2 out of 40 patients, indicating the low immunogenicity of HBM4003. Among PD-1/PD-L1 treatment-naïve patients, the objective response rate (ORR) was 33.3%, rising to 40% in patients with mucosal melanoma, suggesting notable antitumor activity in these subgroups.
A phase II clinical study also evaluated the efficacy and safety of HBM4003 and toripalimab in patients with refractory neuroendocrine neoplasms (NENs) [12]. The combination showed manageable safety, low immunogenicity, and encouraging efficacy. All patients experienced TRAEs, including grade ≥3 in 34.5% with no grade 4/5 irAEs. The ORR was 34.6%, and the disease control rate (DCR) reached 65.4%, with responses observed across multiple NEN subtypes. Overall, HBM4003 plus toripalimab demonstrated promising anti-tumor effects in NEN patients while presenting a manageable safety profile, on par with those generally seen with CPIs.
HBM7008
To address the hepatotoxicity issues associated with urelumab, an anti-4-1BB/CD137 monoclonal antibody, the T cell engager HBM7008 was developed to specifically target the tumor-associated antigen B7-H4 and the costimulatory receptor 4-1BB. Blockade of B7-H4 promotes T cell activation and proliferation, but with low efficacy. Therefore, HBM7008’s dual targeting strategy redirects immune responses to the tumor site while also enhancing anti-tumor efficacy through both mechanisms [13].
The design of HBM7008 leveraged sequences from a fully human IgG anti-B7-H4 antibody (H2L2 Harbour Mice®) and from anti-4-1BB single VH domains sourced from a fully human HCAb (HCAb Harbour Mice®).
Preclinical studies demonstrated that HBM7008 could bind both targets simultaneously, and importantly, T cell activation was dependent on its binding to B7-H4. In in vivo studies using tumor mouse models, HBM7008 promoted potent anti-tumor responses. Significantly, studies in non-human primates with repeated dosing showed that HBM7008 had a favorable tolerability profile, which was instrumental in advancing it to human studies.
Empowering Biotherapeutic Innovation with HCAb Platforms
Nona Biosciences’ fully human HCAb technology unlocks new frontiers in biotherapeutic design, offering VH binders with exceptional stability, solubility, and modularity. Our proprietary HCAb platforms enable rapid generation of high-affinity and diverse binders that can be tailored for a broad spectrum of modalities, including bispecific antibodies, ADCs, CARs, and ICEs. Through integrated discovery and functional screening systems, Nona streamlines the identification of candidates with optimal developability profiles, accelerating the path from Idea to IND (I-to-I®).
Related Resources
To explore the VH architecture and its scientific foundations in more detail:
- View our Infographic on Single Domain Binders for a condensed, visual overview of how fully human VH domains function as modular building blocks across advanced biotherapeutic modalities.
- Check out our Scientific Whitepaper for a deeper technical discussion of fully human VH domains, including developability considerations and early clinical translation.
- Hamers-Casterman C. et al., Nature, 1993. [Link]
- Arbabi-Ghahroudi M. Front Immunol, 2017. [Link]
- Klarenbeek A. et al., Mabs, 2015. [Link]
- Evers A. et al., Mabs, 2025. [Link]
- Rossotti MA. et al., FEBS J, 2022. [Link]
- Fernández-Quintero ML. et al., Protein Sci, 2024. [Link]
- Drabek D. et al., Front Immunol, 2016. [Link]
- Janssens R. et al., Proc Natl Acad Sci U S A, 2006. [Link]
- Kasandra Bélanger et al., Protein Engineering, 2021. [Link]
- Gan X. et al., Proc Natl Acad Sci U S A, 2022. [Link]
- Tang B. et al., J Immunother Cancer, 2024. [Link]
- Zhang P. et al., EClinicalMedicine, 2025. [Link]
- Huang B. et al., J Immunother Cancer, 2022. [Link]