TCR mimic (TCRm) antibodies are an emerging class of therapeutic antibodies that target intracellular tumor antigens presented by MHC molecules, offering several advantages over traditional antibody therapies. These include targeting a broader range of antigens, off-the-shelf drug development, and combining T-cell receptor specificity with antibody pharmacological properties. Compared to traditional TCR therapies, TCR mimic antibodies achieve higher affinities and broader applicability, potentially overcoming limitations such as HLA restriction. Nona Biosciences’ TCR mimic antibodies, in particular, stand out for their high affinity, specificity, and innovative design, positioning them as a promising option in cancer immunotherapy.
TCRm antibodies offer several key advantages over traditional antibody and TCR-based therapies:
TCRm antibodies can recognize peptides derived from intracellular proteins presented on MHC molecules, expanding the range of targetable antigens compared to surface-bound targets of traditional antibodies. This capability allows for identifying and targeting a broader array of disease-associated proteins, potentially leading to more effective treatments.
Through in vitro selection, TCRm antibodies can achieve affinities orders of magnitude higher than TCRs, reaching the subnanomolar range. This increased affinity can potentially enhance their therapeutic efficacy.
TCRm antibodies exhibit several favorable drug-like properties. They possess a long half-life, which allows for sustained therapeutic action. Additionally, they are stable, ensuring consistent performance over time.
TCRm antibodies can mediate tumor cell killing through various antibody-dependent mechanisms like ADCC and CDC. Additionally, they exhibit TCR-like recognition of specific peptide-MHC complexes, providing a multifaceted approach to targeting and eliminating cancer cells.
Compared to TCR-based therapies, TCRm antibodies are easier to produce, making them more accessible for large-scale clinical applications.
TCR mimic antibodies offer several key advantages over traditional antibody and TCR-based therapies:
TCR mimic antibodies can recognize peptides derived from intracellular proteins presented on MHC molecules, expanding the range of targetable antigens compared to surface-bound targets of traditional antibodies. This capability allows for identifying and targeting a broader array of disease-associated proteins, potentially leading to more effective treatments.
Through in vitro selection, TCR mimic antibodies can achieve affinities orders of magnitude higher than TCRs, reaching the subnanomolar range. This increased affinity can potentially enhance their therapeutic efficacy.
TCR mimic antibodies exhibit several favorable drug-like properties. They possess a long half-life, which allows for sustained therapeutic action. Additionally, they are stable, ensuring consistent performance over time.
TCR mimic antibodies can mediate tumor cell killing through various antibody-dependent mechanisms like ADCC and CDC. Additionally, they exhibit TCR-like recognition of specific peptide-MHC complexes, providing a multifaceted approach to targeting and eliminating cancer cells.
Compared to TCR-based therapies, TCR mimic antibodies are easier to produce, making them more accessible for large-scale clinical applications.
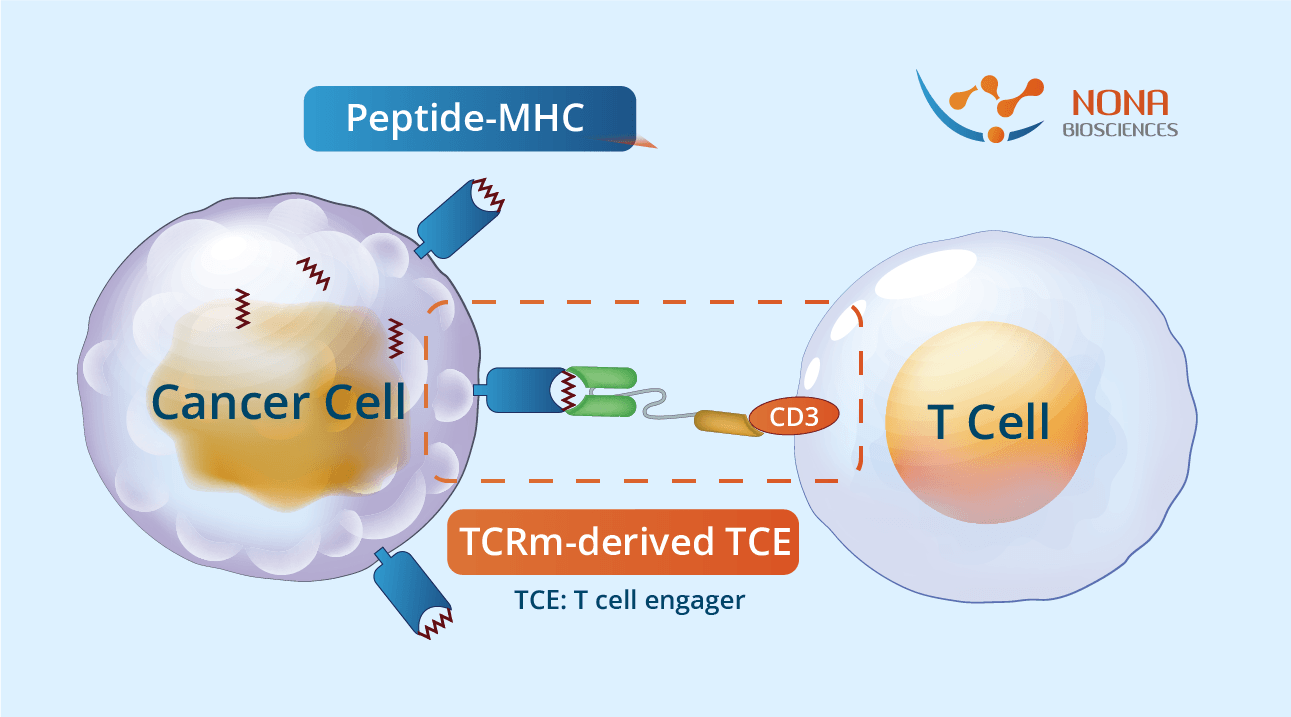
TCRm antibodies can be engineered into bispecific T cell engager (TCE) formats to enhance their therapeutic potential. TCRm-derived TCEs, composed of two linked scFvs, target a TAA and CD3 on T cells, redirecting T cells to kill tumor cells. Incorporating a TCRm antibody as the tumor-targeting component allows TCRm-based TCEs to recognize intracellular antigens presented on MHC molecules, expanding the range of targetable antigens. TCRm-based TCEs engage polyclonal T cells without ex vivo manipulation, offer enhanced tumor specificity due to high affinity and specificity, and induce potent T cell-mediated cytotoxicity against tumor cells expressing low levels of target antigen, highlighting their promise in cancer immunotherapy.


TCR mimic antibodies can be engineered into bispecific T cell engager (TCE) formats to enhance their therapeutic potential. TCR mimic-derived TCEs, composed of two linked scFvs, target a TAA and CD3 on T cells, redirecting T cells to kill tumor cells. Incorporating a TCR mimic antibody as the tumor-targeting component allows TCR mimic-based TCEs to recognize intracellular antigens presented on MHC molecules, expanding the range of targetable antigens. TCR mimic-based TCEs engage polyclonal T cells without ex vivo manipulation, offer enhanced tumor specificity due to high affinity and specificity, and induce potent T cell-mediated cytotoxicity against tumor cells expressing low levels of target antigen, highlighting their promise in cancer immunotherapy.
Nona Biosciences has generated compelling data demonstrating the capabilities and potential of the TCR mimic antibody platform. The TCR mimic antibodies exhibit high affinity and specificity for target antigens, enabled by using high-quality internally generated pMHC and mRNA-encoded pMHC immunogens in their discovery process.
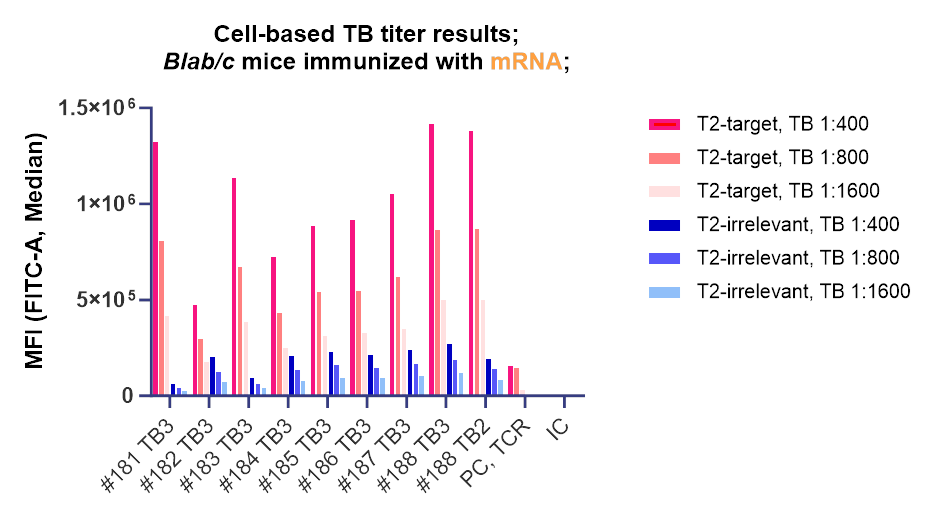
The internally generated mRNA-encoding pMHC immunogen effectively stimulate an immune response
Nona’s TCRm-CD3 engager design, which combines an anti-CD3 nanobody with a TCR mimic Fab and incorporates Fc-silencing modifications, has shown promising therapeutic potential. In preclinical studies, Nona’s TCRm-CD3 engagers demonstrated potent and concentration-dependent cytotoxicity against tumor cells expressing the target antigen, highlighting their efficacy.


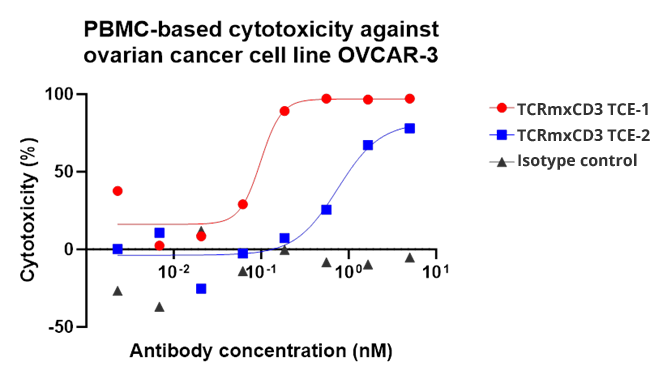

Cytotoxicity of the TCRm-CD3 engagers against OVCAR3 and NCI-H1703 cells indicates that TCRm Abs induce the killing effect on tumor cells.
Furthermore, Nona’s TCR mimic antibodies have achieved subnanomolar affinities for their targets, which are orders of magnitude higher than the affinities of natural TCRs, which typically range from 1-100 μM. This high affinity can potentially enhance the therapeutic efficacy of Nona’s TCR mimic antibodies.



Specific and High Affinity Binding. The identification of TCR mimic antibodies with specific and high affinity to target-pMHC proteins.
Copyright 2026 @ Nona Biosciences. All rights reserved.
Stable cell line
Process development
Manufacture
PK / PD
Efficacy
ADA
TOX
Beacon® single B cell screening
Display technology
CAR-function based functional screening
Hybridoma
HCAb direct cloning screening
TCR Mimic Antibody
Target assessment
Recombinant protein
Recombinant cell-line
Affinity maturation ▶
Humanization▶
Fc-Engineering▶
Structure-Based Protein Design
▶
Functional characterization
Binding / Affinity
Antibody production
In vitro functional assay
Developability
Protein
Cell line
DNA
mRNA
Dr. Tian is an academician of Chinese Academy of Engineering, a member of Academia Europaea and a medical immunologist. Currently, he is a professor at University of Science and Technology of China (USTC), where he also works as Dean at School of Basic Medical Sciences, and Director at Institute of Immunology. He is also the Director of the Key Lab of Innate Immunity and Chronic Diseases of Chinese Academy of Science.
Dr. Tian was awarded with the National Science Fund for Distinguished Young Scholars. He is the academic leader of Chang Jiang Scholars Program as well as the Innovation Research Program of National Natural Science Foundation of China. Dr. Tian is Head of National Science and Technology Major Project and Chief Scientist of National Major Research Plan Program.
Dr. Tian’s laboratory is credited with seminal discoveries regarding basic knowledge and clinical study of natural killer (NK) cells, particularly liver-resident NK cells, cytokine-producing NK cells, and NK cell-based immunotherapy.
Peter Moesta, Ph.D., oversaw the development, production and worldwide launch of important medicines, such as Humira, Yervoy and Opdivo. Dr. Moesta previously served in executive roles at Bristol-Myers Squibb.
Dr. Kramer serves as CSO of Portage Biotech Inc. Dr. Kramer previously served as Vice President and Head of Discovery for Oncology Therapeutics at Janssen Research & Development, LLC (the Pharmaceutical Division of Johnson and Johnson), where he was responsible for leading Global Discovery, focusing on aberrant signaling cascades in tumor cells, as well as epigenetic reprogramming and tumor immunology using both small molecule and protein-based large molecule approaches. Prior to joining Janssen Research & Development, LLC, Dr. Kramer served as VP Drug Discovery and Research for Bristol-Myers Squibb (BMS), where he provided scientific leadership and strategic oversight for many pre-clinical Oncology and Immunology programs and projects that entered development. Dr. Kramer was previously an Assistant Professor at Harvard Medical School. Dr. Kramer received his Ph.D. in pharmacology from the University of Vermont and completed his post-doctoral fellowship in Oncology at the National Cancer Institute, National Institutes of Health.
Dr. Kamen is a Venture Partner at Third Rock Ventures. In 2005, he co-founded BioAssets Development Corporation and served as its Chairman. He currently serves as an independent non-executive Director of Harbour BioMed and a director of Jounce Therapeutics (NASDAQ:JNCE). He was previously a director of Neon Therapeutics and Harbour Antibodies. Earlier in his career, he was senior vice president of scientific affairs at the pioneering biotechnology firm named Genetics Institute, Inc. Dr. Kamen received his bachelor’s degree in biophysics from Amherst College, a Ph.D. in biochemistry and molecular biology from Harvard University Graduate School of Arts and Sciences. During his academic scientific career, he worked at the Imperial Cancer Research Fund.
Dr. Grosveld is Co-founder and CSO of Harbour Antibodies and the inventor of Harbour Mice®, Professor and former Head of Department of Cell Biology and Department of Clinical Genetics at Erasmus University Medical Center, Rotterdam, a fellow of Royal Society and a member of Royal Netherlands Academy of Arts and Sciences. Dr. Grosveld’s research on the control of globin gene expression has been selected as one of the top ten achievements of Medical Research Council (UK) (MRC) in the 20th century by Higher Education and Research Opportunities in the U.K. Dr. Grosveld was awarded the Louis-Jeantet Prize for Medicine in 1991, the Spinozapremie (Spinoza Prize) in 1995.
Dr. Arkinstall has demonstrated remarkable competence throughout his career. He is a respected leader in drug discovery with substantial roles under his belt, including Research Head, Chief Scientific Officer (CSO), and Chief Executive Officer (CEO) positions at various pharmaceutical or biotech companies. Dr. Arkinstall served as CEO of Elstar Therapeutics and Revitope Oncology, companies advancing novel classes of multi-specific antibody-based cancer drugs. He previously was also the CSO of Kymab, an antibody therapeutics company founded in Cambridge, UK, prior to which he spent 16 years in progressively senior research leadership roles at EMD (Merck) Serono, and its associated entities across Europe and the United States.
Dr. Musheng Bao earned his Ph.D. in China and completed his postdoctoral training at MD Anderson Cancer Center and Baylor Institute for Immunology Research. Beginning his professional journey as a Scientist II at MedImmune, Dr. Bao later transitioned to Sanofi, where he served as a Principal Scientist. Following this, he joined Harbour BioMed, where he led a team dedicated to therapeutic antibody development utilizing the Harbour Mice® platform. Presently, Dr. Bao has taken on the role of Head of Biology at Nona Biosciences.
Dr. Yun He is Chief Technology Officer of Nona Biosciences. Before Nona Biosciences spun off from Harbour BioMed, Dr. He served as Head of Antibody Technology at Harbour BioMed. During his tenure there, Dr. He contributed to multiple discovery programs and led the team in establishing HBICE® platform. Prior to joining Harbour BioMed, Dr. He was an Investigator at Biologics Center in Novartis, where he was responsible for antibody engineering and bioinformatics. Prior to that, Dr. He was the group leader of Bioinformatics at GenScript. Dr. He received his Ph.D. from the Chinese Academy of Sciences, with a focus on molecular biology and bioinformatics.
Mr. Louis Liu is Senior Vice President and Head of Scientific Operation of Nona Biosciences. He graduated from Bethune Medical University with Bachelor Degree of Medicine. He has over 30 years’ experience in antibody technology platform establishment, antibody discovery and discovery team management experience. Previously he worked in Syntron Bioresearch Inc as R&D manager, Strategic Diagnostic Inc as product development supervisor, Rockland Immunochemical Inc as Manager of Monoclonal Antibody service and product development, GenScript Ltd as vice president of Antibody Division, Shanghai ChemPartner as vice president of Biologics Discovery.
Dr. Joe Zhao is Vice President and Head of External Innovation of Nona Biosciences. Joe holds a BS from Fudan University and a PhD from University of Delaware, followed by postdoctoral trainings at Lankenau Medical Center and Zeneca Pharmaceuticals. Prior to joining Harbour BioMed, he was in small biotechs (Pharmacopeia and Ligand Pharmaceuticals) and large MNC (BMS). Joe has 25 years of combined experience in drug discovery of both small molecules and biologics in therapeutic areas of immuno-oncology, immunology, and genetic diseases.
Dr. Jiyong Zhang leads business development at Nona Biosciences, bringing vision and dedication to strategic growth and client satisfaction. With 15 years of experience in biotherapeutic research and development, Dr. Zhang’s understanding of market dynamics and ability to identify mission-aligned opportunities are evident.
Before overseeing business development at Nona, Dr. Zhang played key roles at Alexion and Abbvie, contributing to antibody discovery, engineering, and bispecific antibody R&D. His scientific experiences and knowledge in biotherapeutic innovation makes him a forward-thinking strategist focused on enhancing service offerings to meet evolving client needs.
With Dr. Zhang and his team driving business development, Nona Biosciences is poised to offer innovative solutions and unparalleled service. This solidifies the company’s position as a trusted and client-focused drug discovery partner in the dynamic landscape of biotherapeutic innovation.
Dr. Yiping Rong is our Chief Scientific Officer. He is a well-recognized scientist with about 20 years’ experience of biomedical research and drug discovery. Dr. Rong used to work at Sanofi, JNJ and Roche and built strong expertise in cancer biology and pharmacology. He led and contributed to >15 programs entering clinical trials. He is also involved in translational research work for a few drugs. Multiple mAb or bispecific antibodies generated from his team were out licensed to MNCs. Some highly innovative first-in-class projects are in clinical trials. He worked on apoptosis, epigenetics, immuno-oncology, and cancer cell signaling fields and led the drug discovery projects including kinase, enzyme, receptor/ligand, protein-protein-interaction targets by small molecules or monoclonal antibodies. Dr. Rong received his Ph.D. degree in Pharmacology from Case Western Reserve University (Cleveland, Ohio). He is the member of American Association of Cancer Research and has more than twenty publications in cancer research field, including Nature Genetics, Molecular Cell, PNAS papers. He is also the inventor of dozens of patents in drug discovery field.
Dr. Jingsong Wang is Chairman of Nona Biosciences. Prior to that, Dr. Wang served as Head of China R&D and Head of Translational Medicine, Asia Pacific, at Sanofi. He is a former attending physician and clinical fellow at Harvard Medical School. Dr. Wang received his Ph.D. in Molecular Pharmacology from China Pharmaceutical University and has also completed a Molecular Immunology Research Fellowship at Dr. Laurie Glimcher’s laboratory at the Harvard School of Public Health.