Breast Cancer Trends: Incidence and Mortality
Breast cancer remains rare in men but is a top malignancy diagnosed in women and one of the most common causes of cancer-related death in females worldwide [1,2]. A recent analysis by the International Agency for Research on Cancer (IARC) on the burden of breast cancer globally revealed that one in twenty women will receive a cancer diagnosis in their lifetime. Based on current incidence rates, the report provides a staggering estimation of breast cancer-related deaths by 2050 of over 1 million women annually [3].
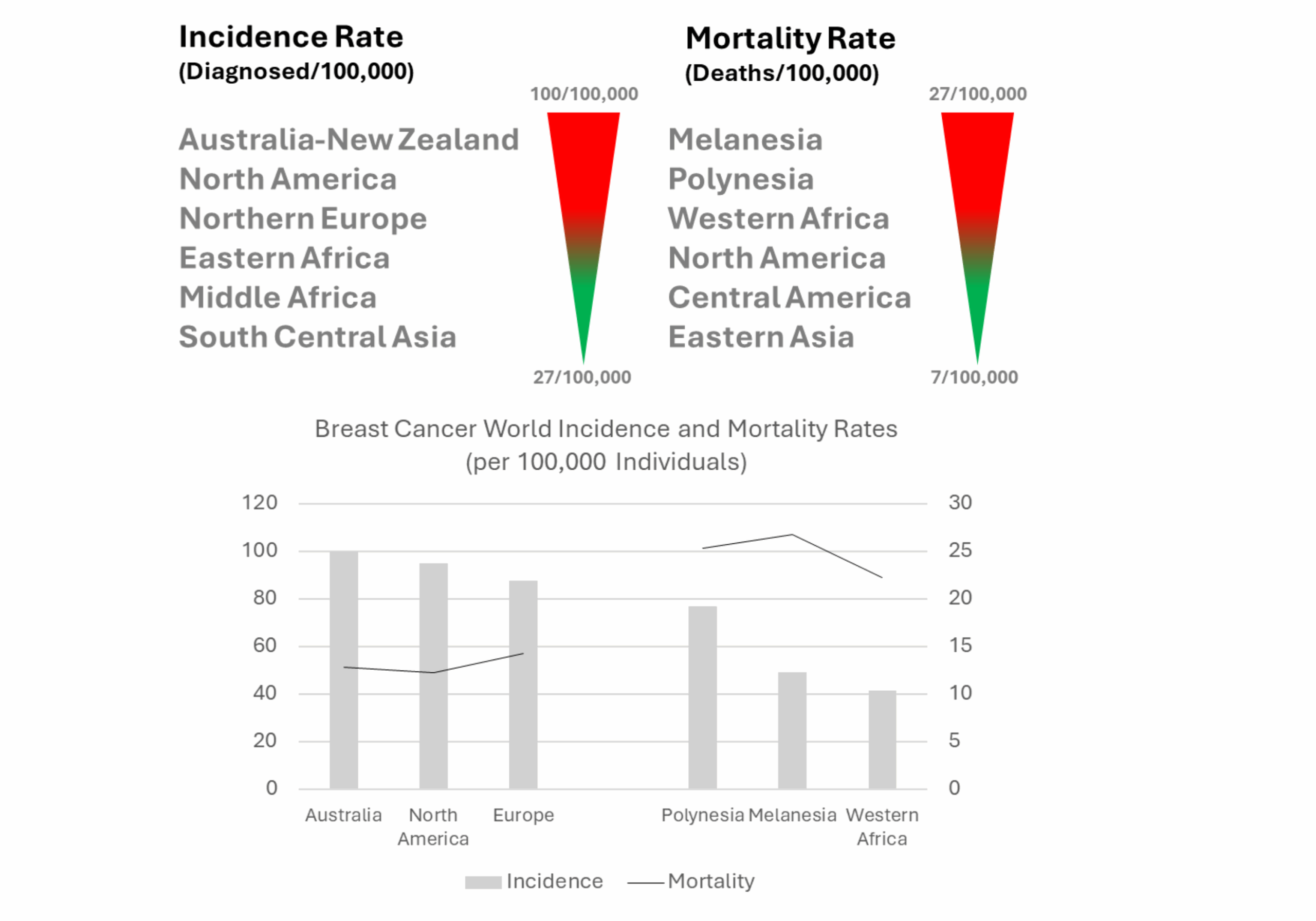
The study analyzed comprehensive data from 50 countries, revealing that the incidence and mortality rates are correlated with the region or country’s “Human Development Index (HDI),” which was established by the United Nations Development Programme (UNDP) and provides a sense of life quality.
For instance, women’s mortality rates could be as high as 56% in countries with a low HDI, compared to 17% in countries with a high HDI, suggesting that limitations in timely diagnosis and treatment are significant factors [3].
Global Breast Cancer Incidence and Mortality by Human Development Index
Complexity of Breast Cancer Diagnosis and Treatment
Breast cancer is a highly complex and heterogeneous disease, necessitating a multidimensional approach to diagnosis and treatment. Its diagnosis requires histological assessment of tumor type and grade, as well as molecular profiling to define hormone receptor status and the expression of critical proliferation markers. Currently, four main subtypes are defined to guide therapeutic course and disease management, including: luminal A (high expression of estrogen receptor- ER, progesterone receptor- PR, human epidermal growth factor receptor-2- HER2, and Ki67); luminal B (hormone receptor positive, Her2+/-, high Ki67), HER2 overexpressing, and triple-negative breast cancer (TNBC, ER, PR and HER2 negative) [4].
Breast cancer treatment can typically involve various approaches, such as surgery, chemotherapy, radiation, endocrine therapy, targeted therapy, and immunotherapy. Disease management is highly dependent on various factors, including the tumor stage and molecular profile, among others.
For instance, hormone therapy and chemotherapy may be selected for fast-growing luminal B tumors, while in HER2-positive breast cancer, a more targeted approach may be preferred [4].
The Impact of Antibody Drugs in Breast Cancer
Antibody drugs have served as powerful tools in the fight against cancer due to their potential for specific tumor targeting, promotion of anti-tumor immunity (e.g., immune checkpoint inhibitors), disruption of proliferation signaling pathways, and delivery of cytotoxic payloads (e.g., antibody-drug conjugates, ADCs).
The use of checkpoint inhibitors, such as anti-PD-L1 antibodies (e.g., pembrolizumab), has shown efficacy in TNBC. Immune checkpoint inhibitors enhance the body’s ability to fight cancer by lifting natural immune restraints, allowing T cells to mount a stronger attack against tumor cells. Clinical trials have demonstrated significant extension of overall survival when combining pembrolizumab with chemotherapy in patients with metastatic TNBC [4].
Benefits of combining immune checkpoint inhibitors and chemotherapy have also been achieved in patients with early-stage TNBC. Additionally, pembrolizumab and chemotherapy prior to surgery have demonstrated to improve outcomes in patients with HR+/HER2− breast cancer [4].
Antibody drugs directed to HER2 have also led to improved outcomes in HER2+ breast cancer. HER2 signaling regulates cellular growth, survival, and differentiation. In HER2-positive breast cancer, receptor overexpression is common, and therefore, targeting cancer cells with HER2 antagonists serves as an effective strategy to block signaling.

To date, three monoclonal antibodies targeting HER2 have been approved for the treatment of breast cancer. Several clinical studies have investigated the use of anti-HER-2 antibodies in conjunction with chemotherapy or endocrine therapy.
Recent clinical trials suggest that anti-HER2 antibodies, such as trastuzumab, may be effectively combined with endocrine therapy or dual HER2 blockade to reduce reliance on chemotherapy in certain breast cancer subtypes [4].
ADCs have emerged as a promising treatment option for breast cancer, with the approval of trastuzumab emtansine (T-DM1) and the more effective trastuzumab deruxtecan (T-DXd) for advanced HER2-positive tumors [4].
ADCs consist of three main components: a targeting monoclonal antibody, a linker, and a cytotoxic payload. As a novel class of therapeutics, ADCs are providing the opportunity to achieve high efficacy in breast cancer by extending the therapeutic window of potent anti-cancer agents.

By leveraging antibodies specific to tumor antigens, ADCs can reduce tumor growth through several mechanisms: ① targeted delivery of potent cytotoxic agents to cancer cells (e.g., topoisomerase inhibitors, microtubule inhibitors), ② silencing of tumor growt-promoting signaling, and ③ activation of anti-tumor immune responses (e.g., antibody-dependent cellular cytotoxicity, ADCC).
Increasingly, ADCs are being evaluated in clinical studies in combination with other immunotherapies to address aggressive and advanced breast cancer subtypes. Recent findings from the DESTINY-Breast09 Phase 3 trial demonstrated that combined treatment with trastuzumab deruxtecan (T-DXd) and pertuzumab leads to a 13.8-month improvement in median progression-free survival compared to the previous standard of taxane, trastuzumab, and pertuzumab [5].
Similarly, the ASCENT-04/KEYNOTE-D19 Phase 3 trial revealed that combining the anti-TRPO2 ADC, sacituzumab govitecan, and pembrolizumab resulted in a 35% reduction in risk of progression or death compared to chemotherapy and pembrolizumab in patients with metastatic PD-L1-positive TNBC [6].
Notably, both studies demonstrated that combined therapies with ADCs maintained a similar safety profile compared to standard treatments.
Overall, these findings underscore the power of ADCs in breast cancer and potentially for other solid tumors, where a fine-tuned cytotoxicity and safety profile is required.
Balancing Efficacy and Safety in ADC Development
Driven by their unique potential, ADC development has experienced significant growth, with over 1,700 drugs being developed primarily for cancer indications [7].
However, developing ADCs is a complex balancing act, as each component must be precisely optimized to achieve the best performance while ensuring safety. From antibody specificity to linker stability and payload potency, subtle design shifts can have a profound impact on safety, efficacy, and manufacturability.
Antibody Selection
Monoclonal antibodies used in ADC development should have high specificity and adequate affinity for their target. First, to achieve optimal ADC performance while minimizing on-target off-tumor toxicity, the monoclonal antibody specificity must be directed toward unique tumor antigens or at least overexpressed tumor-associated antigens (TAAs) that are minimally expressed in healthy tissues [8]. Second, while high affinity is a conventionally desirable property in antibody drugs, ADCs with excessive affinity have an increased tendency to accumulate near blood vessels, thereby reducing tumor infiltration [9]. Therefore, monoclonal antibodies with moderate affinities are desirable, which support both faster cellular uptake and processing of ADCs [9].
The level of target antigen expression is crucial for ensuring the effective binding and internalization of ADCs. Target selection must also prioritize cell surface molecules that are predominantly processed through a lysosomal-targeted pathway [10,11]. This is because, ideally, ADCs should be rapidly internalized and trafficked to the lysosomal compartment, where the payload is released [8].
Lastly, similar to conventional antibody drugs, monoclonal antibodies in ADCs should exhibit high stability, a long half-life, and low immunogenicity to ensure both efficacy and safety [8,9]. To date, most clinically approved ADCs are based on humanized and, to a lesser extent, fully human IgG monoclonal antibodies. Predominantly the IgG1 antibody subtype has been leveraged due to several favorable properties, including its high plasma stability and solubility. Moreover, IgG1 antibodies typically exhibit strong binding interactions with Fcγ receptors, which facilitate eliciting anti-tumor immune responses (e.g., ADCC and complement-dependent cytotoxicity, CDC) [9].
An emerging trend in the field is the use of compact binders, such as HCAb VHH and VH domains, to develop ADCs. This trend is motivated by the low tissue penetration of the larger conventional antibody format (i.e., IgG, ~150 kDa), which significantly limits ADC tumor infiltration to only ~1-2% of the administered dose [12].
HCAb VHH and HCAb fully human VH domains (i.e., Harbour Mice®) not only provide the advantage of smaller size (i.e., ~12- 15 kDa), but also offer exceptional stability, solubility properties, high specificity and affinity, and capacity to bind hard-to-reach targets [12]. A drawback of VHH and VH domains is their rapid renal clearance, which limits their use as a single domain in ADCs. However, preclinical studies are underway using these building blocks to generate VHH and VH multidomain-based ADCs (e.g., PSMA-VH x PSMA-VH x HSA-VH and EGFR-VHH x Protein MRI Contrast Agent x HSA-VHH) with improved plasma retention [12,13,14].
Linker Selection
Linkers serve an essential function in ADCs by supporting the covalent binding of payloads to monoclonal antibodies. Because linkers are critical for the effective delivery of payloads to target sites, they must exhibit high stability in circulation. However, linker design must also enable the rapid release of the payload once ADCs are internalized and within the lysosomal compartment.
Non-cleavable linkers are designed to withstand extracellular conditions and are only sensitive to lysosomal compartment elements (i.e., low pH and enzymes), ensuring the release of cytotoxic payloads intracellularly.
To expand upon the ADC’s mechanism of action, antibodies may be conjugated to payloads through cleavable linkers, which are engineered to exhibit specific sensitivities to extracellular and intracellular factors (e.g., enzymes, pH, and reducing conditions) [10].
For example, cleavable linkers may be broken down by proteolytic enzymes within the tumor microenvironment, promoting a bystander effect (i.e., killing of cancer cells not directly targeted by the ADC). Cleavable linkers have been utilized in the design of the majority of approved ADCs (over 80%) [15].
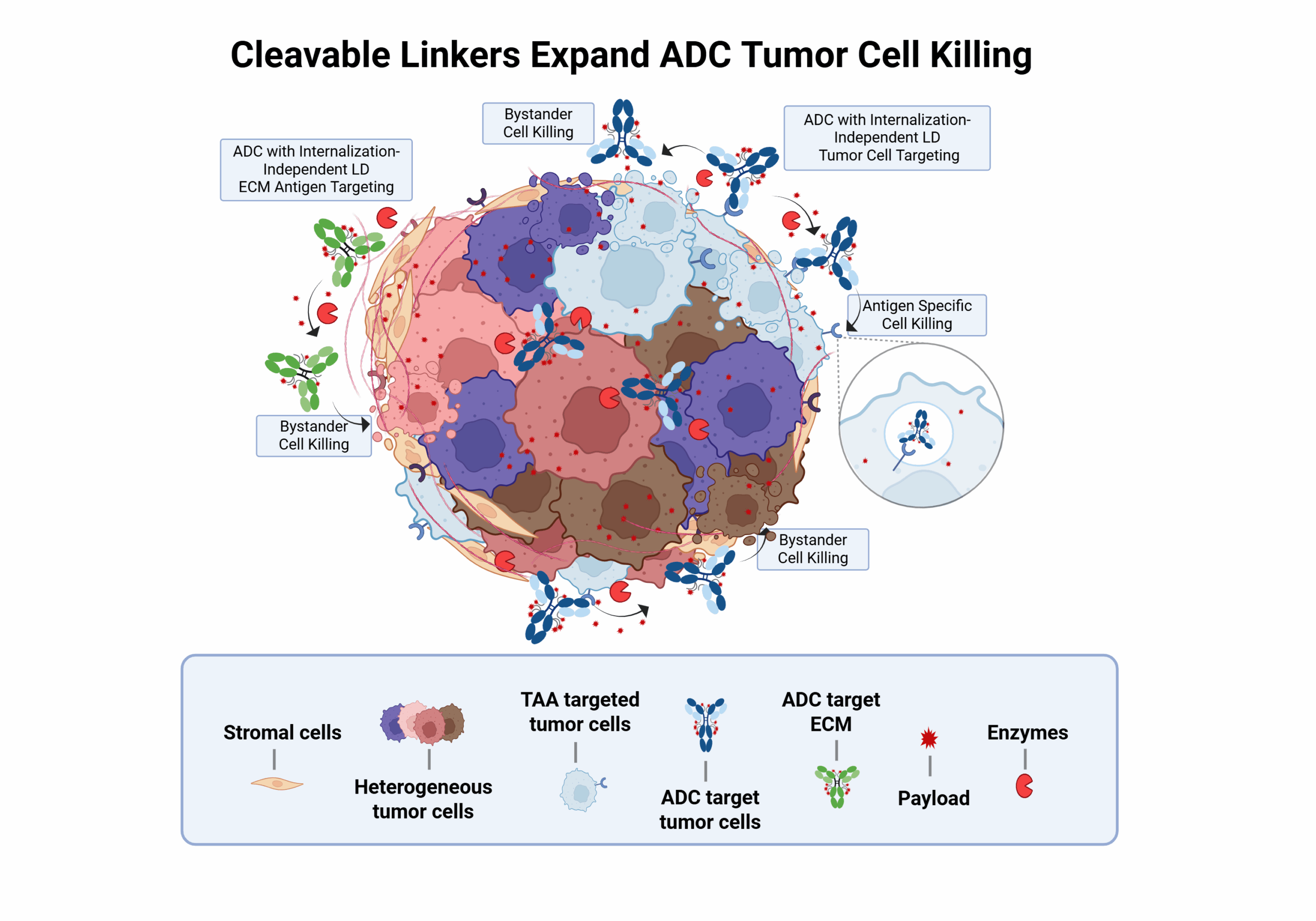
Optimal linkers ensure the efficacy and safety of ADCs. However, linker optimization is complex, as it requires fine-tuning various elements, including conjugation to the antibody and the payload, as well as the chemistries enabling payload release.
Linker coupling to the antibody is particularly critical to ensure homogeneous and effective ADCs. Coupling can be non-site-specific (i.e., to lysine or cysteine residues) or site-specific (i.e., to engineered antibody modifications, such as cysteine or unnatural residues) [9]. The coupling method selected determines the drug antibody ratio (DAR) of the final ADC and ultimately its therapeutic potential [9]. Site-specific coupling strategies have been most commonly used in approved ADCs as they enable more consistency in payload coupling and, ultimately, more homogeneous drugs [9,12].
Lastly, linker hydrophilicity is a crucial attribute requiring careful optimization. Linkers with low hydrophilicity can adversely affect payload conjugation, resulting in low DARs. Moreover, low hydrophilicity can also impact the overall solubility, pharmacokinetics, and safety profile of ADCs [15].
Payload Selection
ADC payloads are critical determinants of therapeutic efficacy and, therefore, require careful selection and optimization to balance potency, stability, and safety. Most payloads are highly cytotoxic agents, such as microtubule inhibitors or DNA-damaging compounds, chosen for their ability to kill cancer cells at low concentrations. However, their extreme potency, commonly 100 times more toxic than systemic chemotherapeutics, also poses risks of off-target toxicity if released prematurely or inadequately targeted [15].
One major challenge is ensuring that the payload remains stable in circulation while being efficiently released intact into the lysosomal compartment after internalization. To achieve this, in addition to linker optimization, the hydrophobicity of payloads must be optimized [9]. Payload hydrophobicity can lead to aggregation or unfavorable pharmacokinetics, necessitating chemical modifications to enhance solubility and mitigate immunogenicity.
The drug-to-antibody ratio (DAR) also plays a pivotal role; too few payloads may reduce efficacy, while too many can compromise antibody structure and increase toxicity.
Moreover, the payload must be compatible with the internalization and trafficking behavior of the target antigen to ensure effective delivery. Resistance mechanisms, such as payload efflux pumps or reduction of its internalization, can also limit payload effectiveness and must be considered during design [15].
Emerging payload classes, including immune modulators, radioisotopes, and targeted protein degraders, are offering new therapeutic avenues [12].
Criteria and Challenges for ADC Optimization
| ADC Component | Optimization Criteria | Key Challenges |
|---|---|---|
| Monoclonal Antibody |
|
|
| Linker |
|
|
| Payload |
|
|
Key optimization criteria and challenges for each ADC component, including antibody, linker, and payload considerations.
Advancing ADC Development with Nona Biosciences
Nona Biosciences addresses challenges in ADC development through its comprehensive antibody discovery and development platforms based on Harbour Mice®. These platforms excel at identifying and engineering fully human antibodies with exceptional specificity and optimal affinity for tumor-associated antigens in breast cancer, laying a solid foundation for creating effective ADCs.
Beyond antibody generation, Nona Biosciences offers end-to-end solutions including conjugation strategies, payload selection, and analytical characterization. This integrated workflow ensures optimal linker-payload design, stability, and manufacturability—key factors in achieving potent anti-tumor activity while minimizing systemic toxicity.
- Li N. et al., Exp Hematol Oncol, 2025. [Link]
- International Agency for Research on Cancer, World Health Organization, Global Cancer Observatory, n.d. [Link]
- International Agency for Research on Cancer, World Health Organization, IARC Reports, 2025. [Link]
- Xiong X. et al., Sig Transduct Target Ther, 2025. [Link]
- ASCO, Daily News, n.d. [Link]
- ASCO, Daily News, n.d. [Link]
- Globe Newswire, Press Release, 2025. [Link]
- Shivatare VS. et al., Isr J Chem, 2023. [Link]
- Wang R. et al., J Hematol Oncol, 2025. [Link]
- Jin S. et al., Sig Transduct Target Ther, 2022. [Link]
- Esapa B. et al., Cancers (Basel), 2023. [Link]
- Medina Pérez VM. et al., Cancers (Basel), 2024. [Link]
- Nessler I. et al., Cancer Res, 2020. [Link]
- Huang H. et al., Chem Commun, 2019. [Link]
- Su Z. et al., Acta Pharm Sin B, 2021. [Link]